Introduction
Emicizumab is expected to provide hemostasis equivalent of 10-20% FVIII activity. Major surgical procedures therefore require additional replacement therapy. A defined protocol of replacement therapy for peri-operative hemostasis in patients with hemophilia A on emicizumab prophylaxis undergoing elective major surgery was prospectively evaluated.
Patients and methods
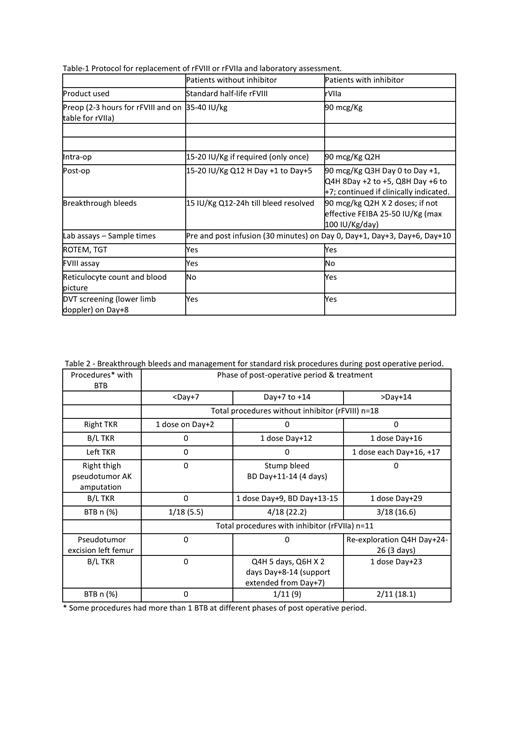
In this ongoing investigator-initiated study of peri-operative hemostasis in patients with severe haemophilia A with or without inhibitors, aged 2 - 60 years on emicizumab prophylaxis for at least 4 weeks, those requiring elective major surgery were evaluated. Additional hemostasis was provided through standard half-life rFVIII (those without inhibitors) or rFVIIa (those with inhibitors) based on a defined protocol (Table 1). Replacement therapy was stopped after 5 or 7 days in those without or with inhibitors, respectively, unless otherwise indicated. No thromboprophylaxis was used. Outcome measures included efficacy of surgical (post-op assessment by surgeon) and post-operative hemostasis (assessed post wound healing by hematologist), as per ISTH SSC criteria. Other parameters monitored included blood counts, FVIII level, FVIII like activity, modified one stage FVIII assay with emicizumab calibrator, ROTEM, TGT and doppler screening of lower limbs for DVT at defined times.
Results
Thirty patients (12 with inhibitors), median age 30.5 years (range: 14 - 56) who underwent 32 surgical procedures were included in this analysis. There were 25 (78.1%) orthopedic, 4 (12.5%) abdomino-pelvic, 1 (3.1%) thoracic, 1 (3.1%) neck and 1 (3.1%) inner ear procedures. These included 3 long standing giant body cavity pseudotumors - 2 abdomino-pelvic and 1 thoracic - which were analysed separately as high-risk for surgical hemostasis.
Hemostatic efficacy for the 29standard risk procedures was assessed as excellent in 27/29 (93%) and good in 2/29 (7%). It was assessed as fair in 2/3 (67%) and poor in 1/3 (33%) high risk cases. The overall hemostasis at wound healing was assessed as excellent in 28/29 (97%), good in 1/29 (3%) for standard risk procedures and good in 2/3 (67%) and moderate 1/3(33%) for high- risk procedures. Pre-op ROTEM and TGT parameters were normal / near normal after rFVIII or rFVIIa infusion.
Breakthrough bleeds (BTB) occurred in a total of 7/29 (24.1%) standard risk procedures, neither requiring additional transfusion support nor affecting wound healing in 6 of them. All BTBs occurred only in total knee replacement (2 unilateral, 3 bilateral) and 2 large thigh pseudotumor excisions. In all except 1, these BTBs occurred after stopping rFVIII/rFVIIa 5- or 7-days post-op. BTBs occurred within day+14 (3 without & 1 with inhibitor) in 4 procedures & beyond day +14 after wound healing (3 without & 2 with inhibitors) in 5, during physiotherapy and mobilization, with some occurring in both phases in 3 procedures. (Table 2)
In 2 patients with early BTB after bilateral TKR, the replacement was extended (rFVIIa Q4H from day +8 to day +12, Q6H from day +13 to day+14; & rFVIII Q12H from day +13 to day +15 and single doses on day+9 and day+29). Two patients with lower limb pseudotumor resections required additional support: 1 from day+11 to day +14 (rFVIII Q12H, 4 days for bleeding during physiotherapy) & the other from days +24 - +26 (rFVIIa Q4H, 3 days for re-exploration of a superficial hematoma).
For the 3 high-risk patients who underwent abdomino-pelvic or thoracic giant pseudotumor resections (2 without inhibitor & 1 with inhibitor), the rFVIII / rFVIIa support was electively continued till day +21 in 2 and day+21 - 32 in 1. There were multiple BTB requiring additional transfusion of blood products (2 without inhibitors received only packed red cells) while the one with inhibitor had continued bleeding even with global hemostasis parameters in the normal range. This continued post-operatively with septic complications leading to death on day +23.
There was no thromboembolism or thrombotic microangiopathy in any patient.
Conclusions
We have demonstrated a safe and effective protocol for standard risk major surgeries for hemophilia A patients with or without inhibitors on emicizumab prophylaxis with additional replacement therapy during the first 5-7 days post-op. Extended support needs to be individualized as per the surgical procedure and post-op care.
Acknowledgements
Supported by Roche. Novo Nordisk provided rFVIIa.
Disclosures
Abraham:Roche: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Pfizer: Research Funding. Mathews:GSK: Honoraria. Srivastava:Octapharma: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biomarin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spark: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal